This is the second installment on our monthly series discussing the science of Alzheimer’s disease research and describing the work we are doing at Scripps Florida to develop effective treatments.
Lesson 2.
Q. What causes the increased beta-amyloid levels that trigger Alzheimer’s disease?
A. An imbalance between beta-amyloid production and clearance.
Last month, I introduced the main culprit in Alzheimer’s disease—beta-amyloid—a small protein fragment that has a strong tendency to stick to itself, forming aggregates that are highly toxic to neurons (nerve cells) and eventually form the plaques that litter the brains of Alzheimer’s patients. This month, I’m going to discuss two different approaches that are being developed to reduce the levels of beta-amyloid in the brains of Alzheimer’s patients.
At this point, I would like to introduce an analogy between Alzheimer’s disease and something we can all understand—an overflowing kitchen sink. In this analogy, beta-amyloid is represented by the water, the brain is represented by the sink, and the disease (too much beta-amyloid) is represented by the flooding that occurs when the sink overflows.
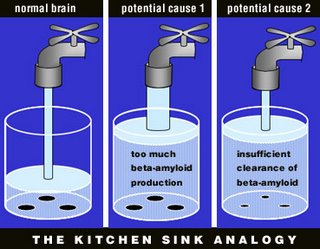
By way of this analogy, we can see that there are two potential causes for the disease (flooding)—too much beta-amyloid production (the faucet is turned up too high), or insufficient clearance of beta-amyloid (the drain is clogged). Likewise, there are two potential treatments: lower beta-amyloid production (turn down the faucet) or increase its clearance (unclog the drain).
Until recently, almost all Alzheimer’s disease research was focused on the faucet—the enzymes that produce the beta-amyloid fragment by cutting it out from its precursor molecule. These enzymes are known as “secretases,” and there are two of them: beta-secretase, which makes the first cut, and gamma-secretase, which makes the final cut. Gamma secretase is especially important, because it is a somewhat “sloppy” enzyme that is capable of cutting at different sites, thereby producing beta-amyloid fragments of different lengths. This point is critical, because longer beta-amyloid fragments are much more prone to clump together and much more toxic, whereas some evidence suggests the shorter forms might actually be protective. This is similar to the concepts of “bad cholesterol” and “good cholesterol.”
Work in my lab (the Leissring lab) is focused on the drainage system, particularly a different set of enzymes that destroy beta-amyloid by cutting it into pieces. We were the first to show that increasing the activity of these enzymes can completely prevent Alzheimer’s disease in mouse models (Leissring et al., Neuron 2003), providing an essential proof that this approach may work in man.
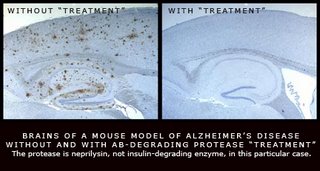
There are several aspects of the “unclogging the drain” approach that are particularly exciting from a therapeutic perspective. There are many more enzymes involved in beta-amyloid clearance, and targeting any one of these enzymes can effectively treat the disease—as our work has shown (Leissring et al., Neuron 2003). More enzymes means more drug targets, thus greatly increasing the chance that at least one drug can be found to safely treat the disease.
Intriguingly, by targeting the drainage system instead of the faucet, it is possible to develop treatments that don’t even need to enter the brain. This idea is based on the recent revelation that beta-amyloid can go back and forth between the brain and the blood stream. Our group has been exploring the novel idea that clearing beta-amyloid from the blood stream can lower beta-amyloid in the brain, and this idea is just now proving to be correct in animal models of the disease. By contrast, drugs targeting the secretases involved in beta-amyloid production (the faucet) are absolutely required to enter the brain, because that is where most beta-amyloid is produced. Getting drugs into the brain is not only difficult, but also carries many risks, so this new approach is very exciting.
As you can see, we have our work cut out for us—we are working on an exciting new approach to treating Alzheimer’s disease, one that involves far more potential drug targets than the two involved in beta-amyloid production, which themselves have dominated Alzheimer’s drug development for the past 10 years. The potential of this new approach has barely been tapped, with many novel drug targets awaiting therapeutic evaluation. At Scripps Florida, we have drug-screening capabilities on a par with large pharmaceutical companies, which is very unique for an academic institution. By contributing to The Unforgettable Fund, donors can help to advance this promising new research avenue, which has great potential for discovering novel and truly disease-modifying therapies for this devastating disease.
Reference:
Leissring MA, Farris W, Chang AY, Sun X, Walsh DM, Frosch MP & Selkoe DJ. Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, associated pathology and premature death. Neuron 40:1087-93, 2003.

No comments:
Post a Comment